The Solution
A Simple Strategy for a Complex Problem
CAUTI & IAD prevention in acute care settings is possible, effective, and increasingly urgent. The most effective way to prevent CAUTI & IAD is to avoid unnecessary use of urinary catheters and absorbent products, and instead prioritise appropriate alternative solutions tailored to the patient’s condition. The solution lies in a multi-level approach that infection prevention programs that prioritise alternative solutions.
The Solution
Approaches to urine management in Acute Care.
Effective Prevention programs
APIC guidelines March 2025 provide information and tools to reduce the risk of infection due to indwelling urinary catheters in various practice settings.
CAUTI Effective prevention programs
IAD Effective prevention programs
Alternative solutions to UIC and Absorbent pads in acute care settings programs
External Urinary Collection Device Systems (EUCDs)
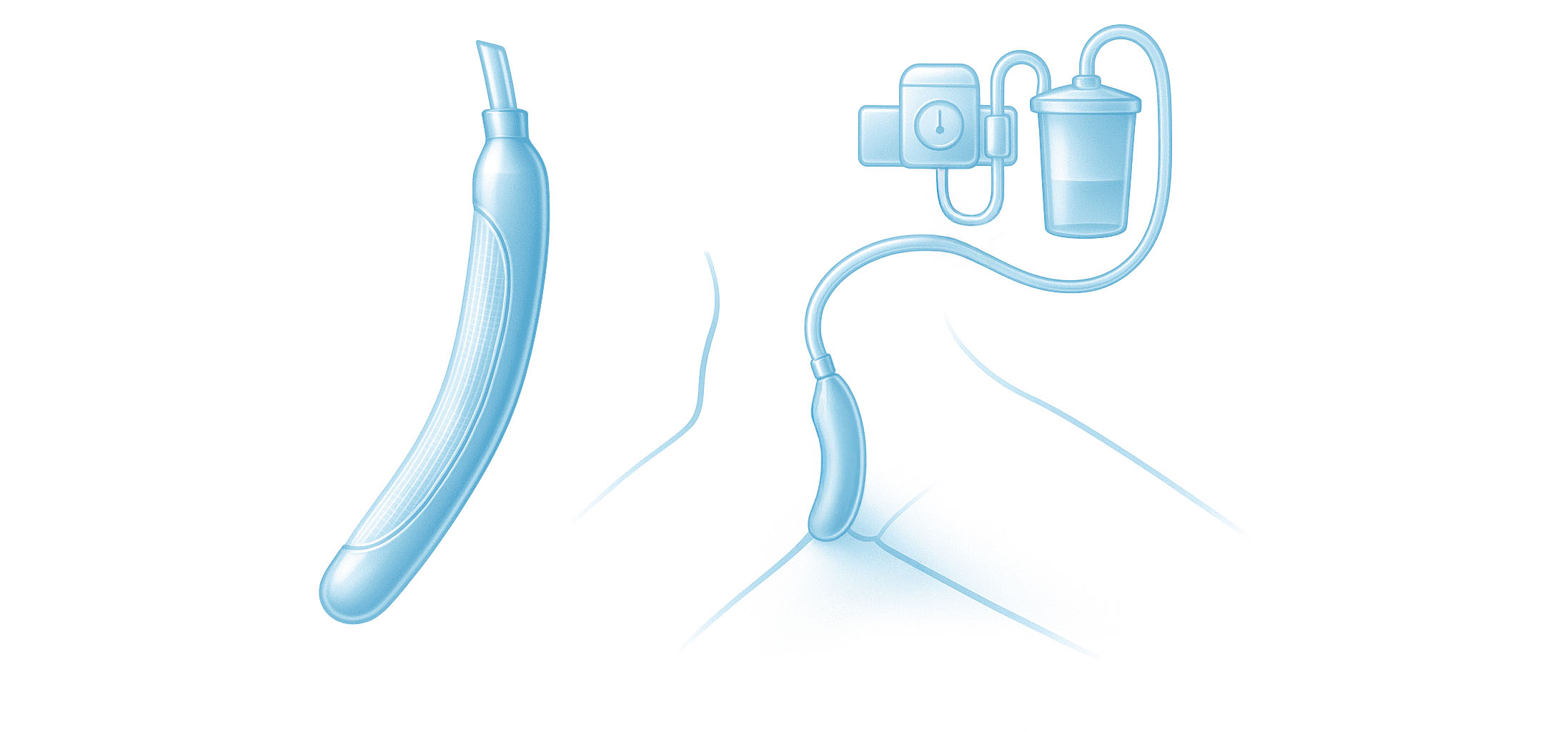
An external urine collection device (EUCD) is defined as a catheter or product that attaches to the perineum. These collection systems drain urine via tubing attached to a bag or via tubing that suctions urine to a container. EUCDs are primarily used in men or women with urinary incontinence. They are either one-time disposable devices or reused multiple times and are made from many materials but the most common material is latex and silicone.
For Women
For Men
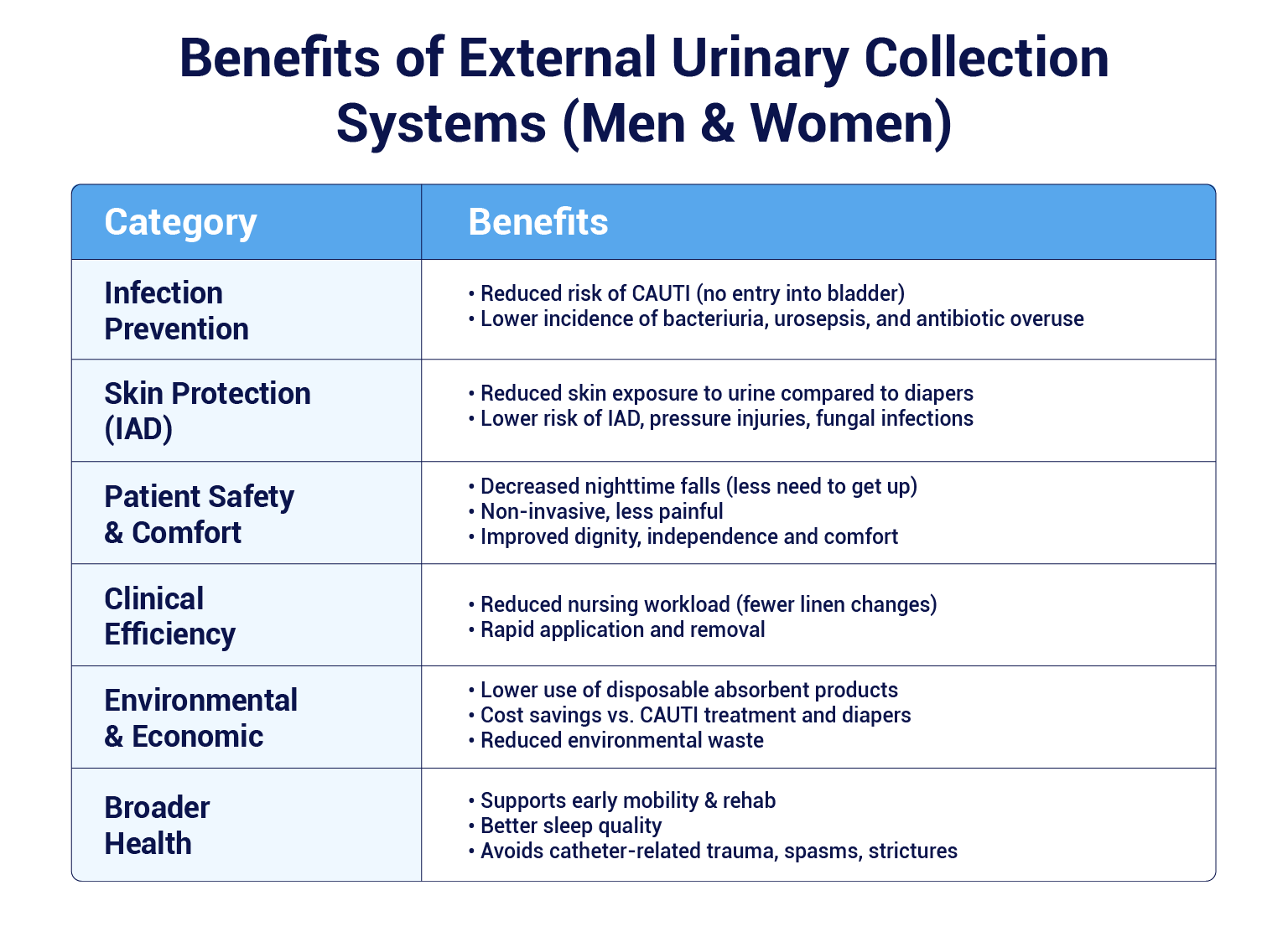
Benefits associated with EUCDs
CAUTI Effective prevention programs (APIC guidelines 2025):
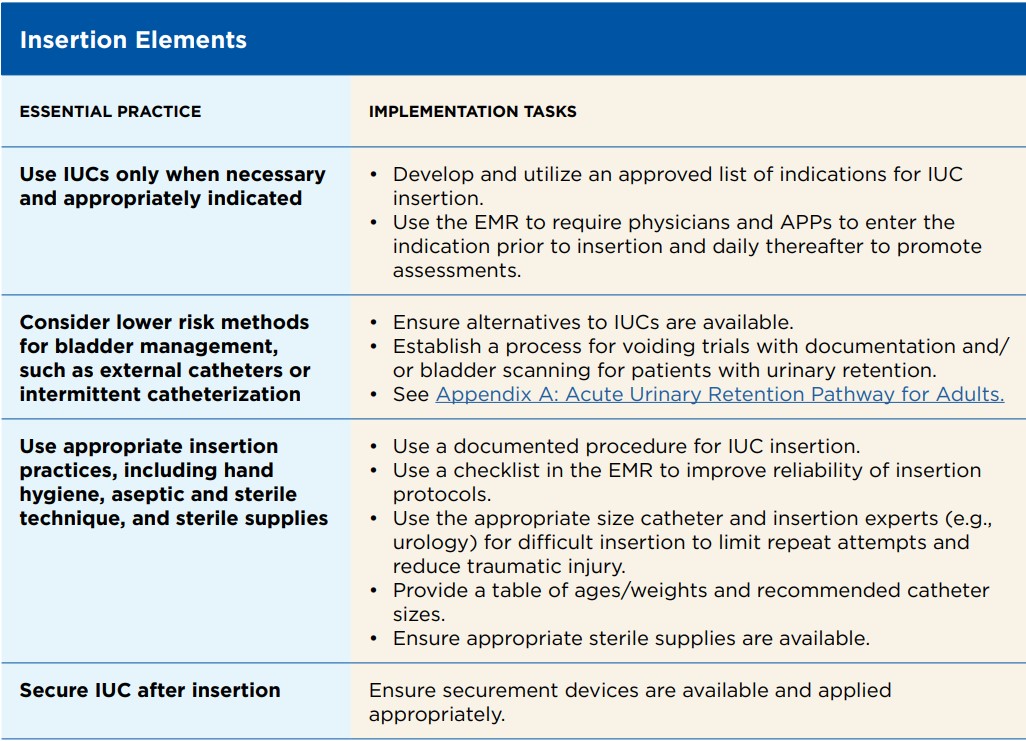
— Not inserting an IUC unless strict criteria are met (e.g., neurogenic bladder, obstructive uropathy)
— Using external urinary catheters when appropriate for the patient
— Limiting the duration of the IUC by using facility-specific removal criteria
— Following aseptic techniques for insertion and maintenance of IUC
Equipment
& supplies
Training and education
Policies and procedures
Information Technology
Equipment
& supplies
Training
and education
Policies and procedures
Information Technology
Facilities must ensure the appropriate equipment and supplies are available to support CAUTI prevention practices. These supplies should be well organized and easily accessible, and staff should be trained on appropriate selection and use based on the needs of each patient. The information below outlines options for supplies and considerations for determining if they are appropriate for the facility:
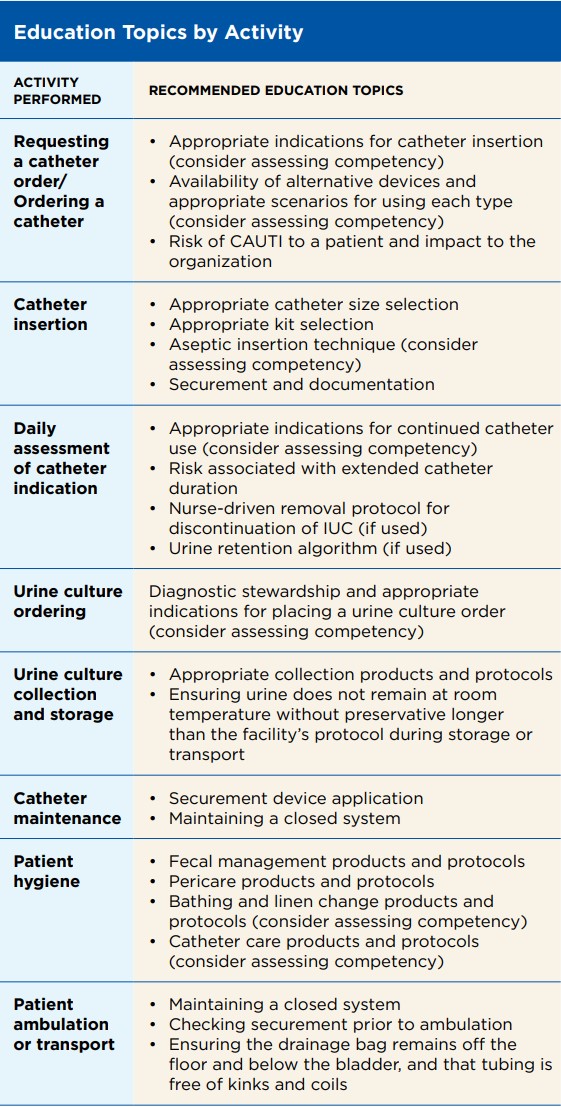
Training and education are vital components of a CAUTI prevention program and should be addressed upon hire and at least annually with updates as needed. The facility should identify who will be responsible for each part of the education and competency process, including:
1. Identification of roles requiring education only versus both education and competency validation
2. Development of rolespecific education and/or competency validation
3. Administration and documentation of education
4. Assessment and documentation of competency
Policies and Procedures A CAUTI prevention policy is a key tool in communicating expectations for CAUTI prevention practices across the facility. The foundation of a policy should be well-recognized EBGs, and care should be taken to avoid including elements that may routinely change over time (i.e., referencing product brand names). During regulatory and accreditation surveys, the facility should be in compliance with each element of its policy, therefore it is important to consider the wording and content included. Procedures provide more detailed information instructing the end-user on how to complete a task and can supplement a policy as needed. Below is one example of how each type of document might be leveraged to provide the right amount of detail for use. Checklists can also be created to summarize key steps for procedures.
Essential Practices
Essential practices, which may be included within bundles, that are widely considered essential for preventing CAUTI are:
Electronic technologies can be leveraged as part of a CAUTI prevention program to enhance accuracy, efficiency, and reliability. Below are considerations for each type of technology as it relates to a prevention program:
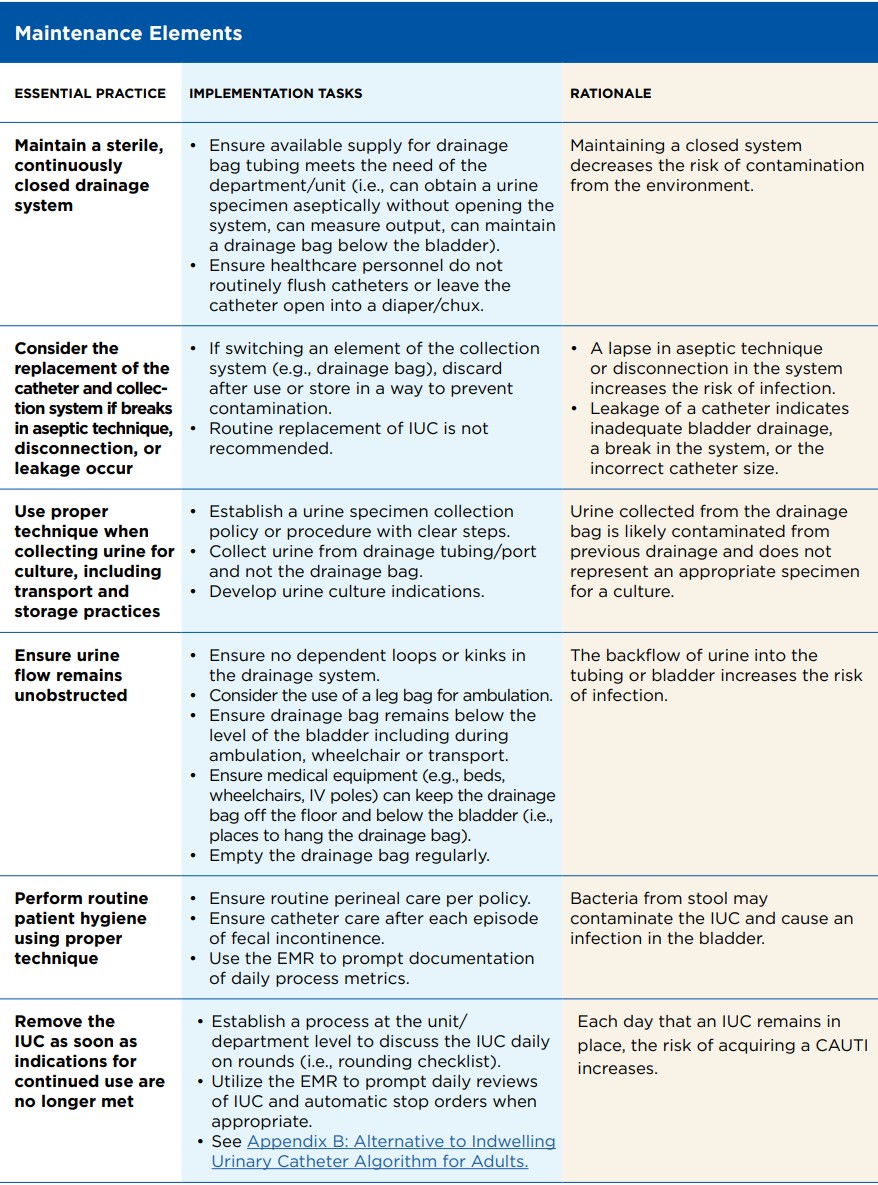
— Electronic medical record (EMR) software: As EMR software continues to evolve, infection preventionists should routinely reassess ways the technology can be leveraged to promote compliance with best practice. When changes to the EMR are considered, collaborating with clinicians is critical before adopting the changes to workflows.
-
-
Providing decision support and guidance for clinicians Example: Modifying urinalysis and culture order workflows within the EMR to encourage use of the most appropriate laboratory test for patients with an IUC
-
Providing prompts and alerts for high-risk scenarios Example: Building alerts in the EMR that notify clinicians when pericare has not been documented within the required timeframe according to the facility policy.
-
Monitoring compliance with documentation of prevention practices Example: Creating monthly reports that summarize the frequency in which patients with an IUC had pericare documented according to the facility policy
-
— Infection prevention surveillance software: Multiple surveillance software products are available, each with a focus on improving efficiency and reliability of data. Each facility should assess its internal needs and resources when determining which surveillance software is appropriate. A cost/ benefit analysis can be useful in describing the potential time savings associated with introducing surveillance software and how that software could assist infection prevention and control (IPC) in reallocating time towards more effective workflows.
— Dashboards and reporting software: Many facilities utilize software to display the current status of key outcome and process measures. Infection preventionists should work closely with the information technology (IT) and quality departments to ensure that IPC metrics are accurate and communicated widely to all relevant stakeholders.
Outcome metrics
Reflect the impact of a healthcare service or intervention on the health status of patients.These measures result from numerous factors, some beyond the control of the healthcare facility. The following are commonly used outcome metrics for CAUTI:
For men
There are several type of systems for men:
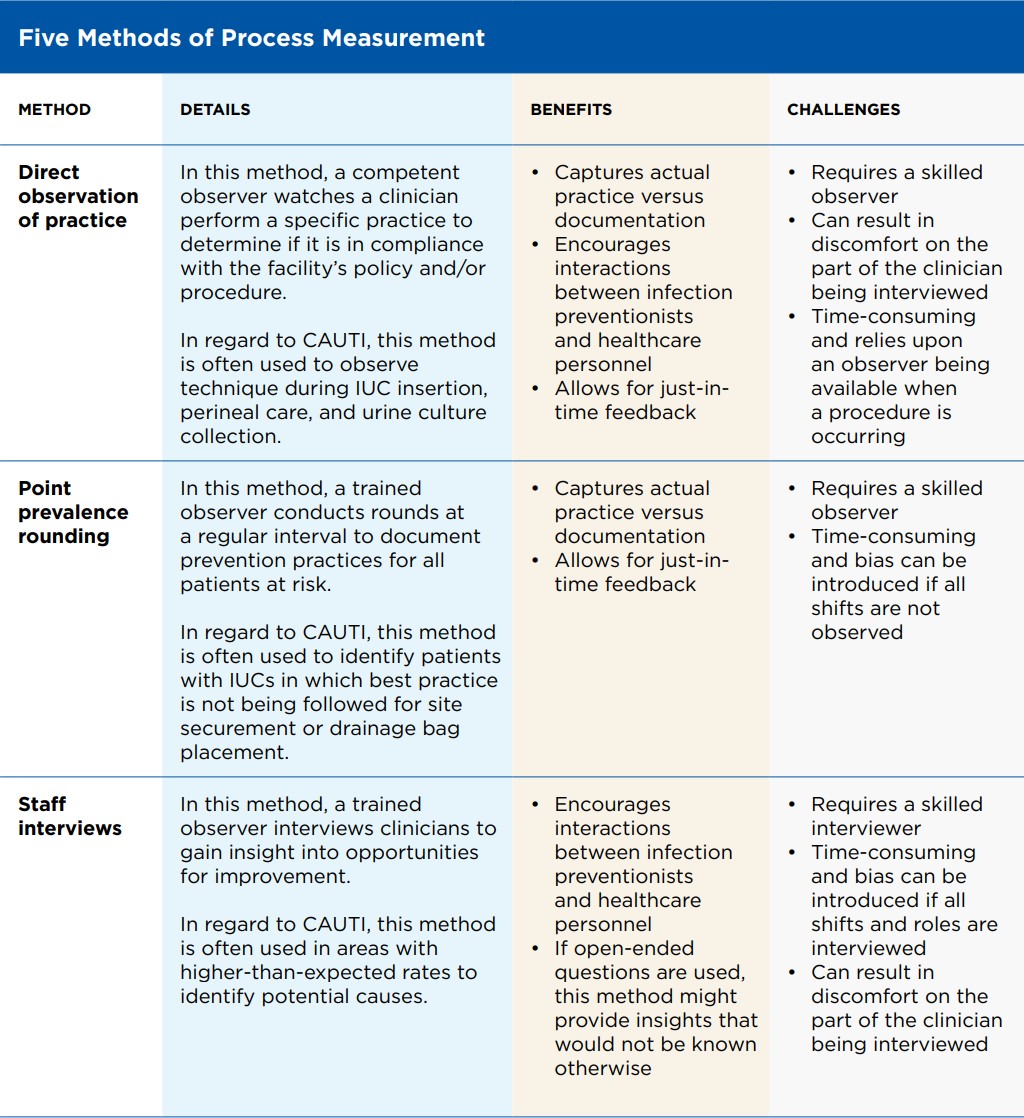
1. Condom Catheters (Sheath Catheters)
-
A sheath, similar to a condom, is rolled over the penis.
-
It has an outlet that connects via tubing to a urine collection bag.
-
Materials: silicone (most common, hypoallergenic), latex (less used now due to allergy risk).
-
Fixation: adhesive strip, self-adhesive sheath, or strap.
2. Men’s Urine Pouches
-
Instead of a condom-like sheath, a soft pouch surrounds the penis and collects urine.
-
Often designed for men with retracted penis or when a condom catheter does not adhere well.
-
Typically secured with an adhesive hydrocolloid baseplate to the perineum.
3. Male External Wicking Systems (less common than in women)
-
Work similarly to female systems, but for men.
-
A wick or cup-shaped device is placed against the urethral meatus/perineum.
-
Connected to a low-level suction system that continuously draws urine away into a canister.
-
Approximately 30% of men have incompatible anatomy for condom catheters, leaving nurses without good alternatives for these patients. These systems are one alternative for nurses for those patients.
-
Skin integrity: Devices must be applied carefully to prevent skin breakdown.
-
Fit: Correct sizing is essential (especially with condom catheters).
-
Hygiene: Daily cleaning and replacement are required to reduce skin irritation and infection risk.
-
Suitability: Not ideal for men with severe urinary retention, bladder outlet obstruction, or very frequent voiding at high flow rates.
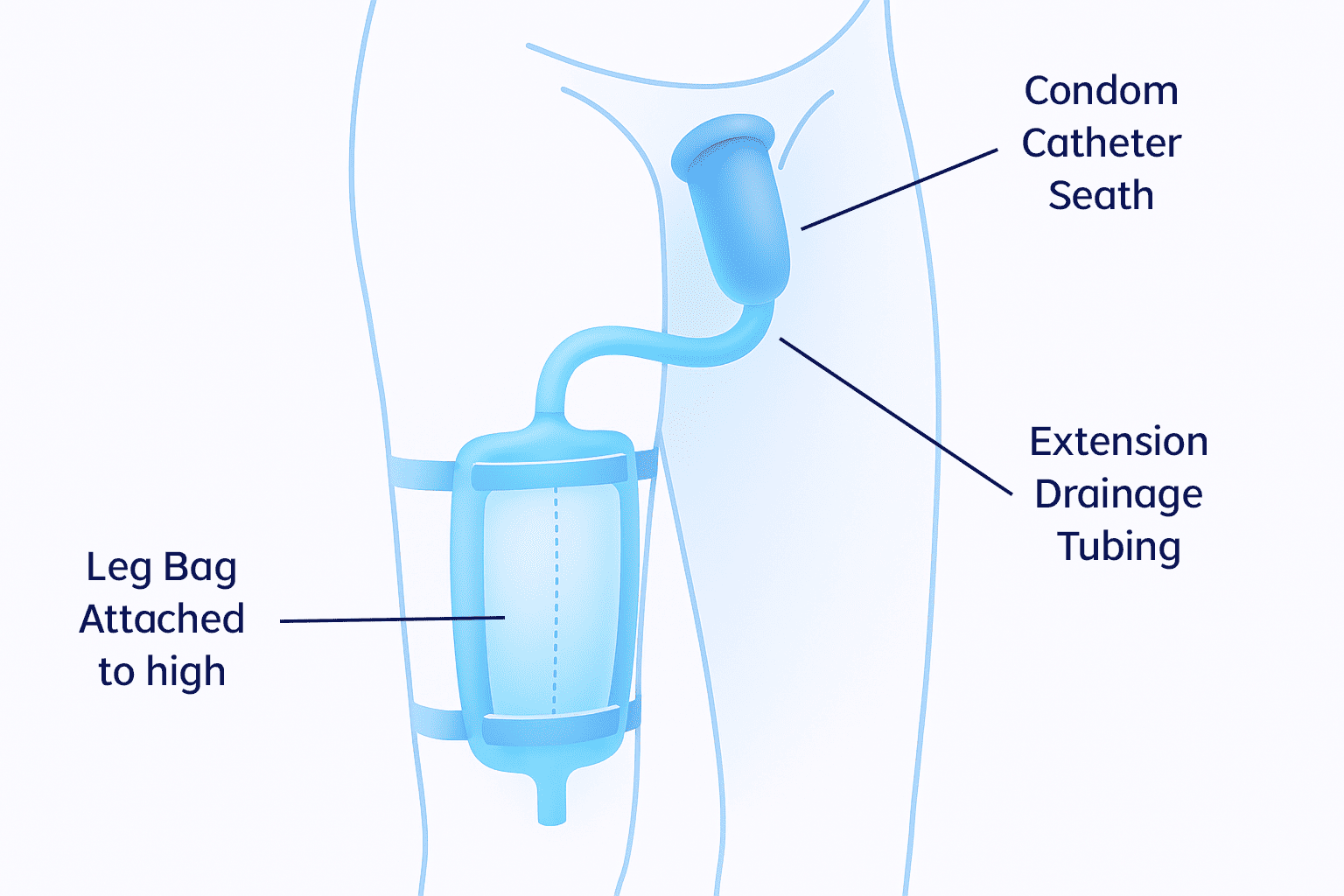
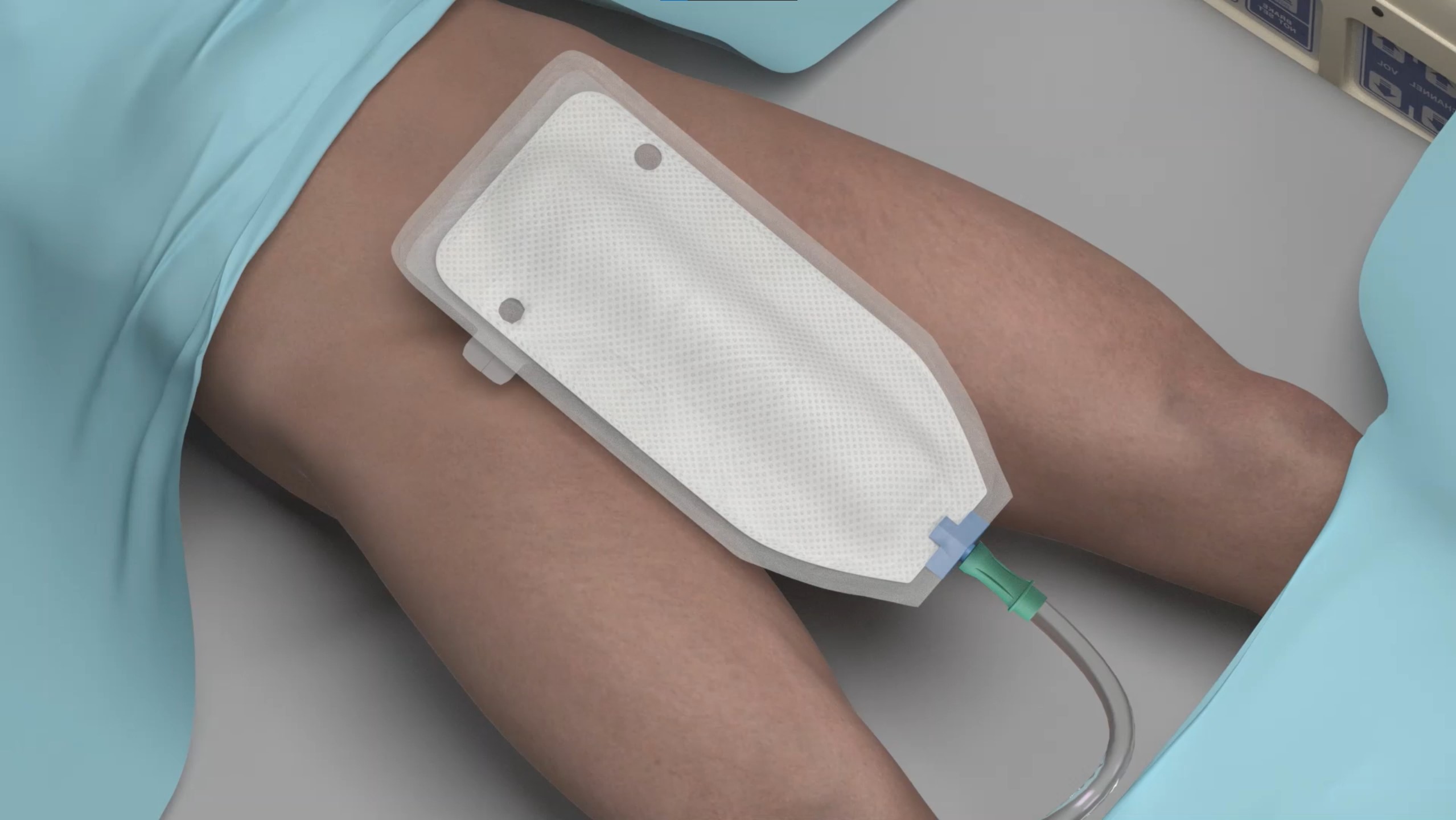
For women
- Easily positioned between the labia, with the top end of the device aligned with the pubic bone and the other tucked gently between the gluteus muscles
- Low-pressure suction wicks urine away from the patient through soft material on the patient-facing side of the device
- The soft, flexible device stays comfortably in place without the need for tape or adhesives that may impact skin integrity
Benefits associated with EUCDs
EUDCs are designed to avoid invasive urethral catheterisation, when clinically not required, thereby lowering the risk of CAUTI .