The Problem
Urine management represents a challenge for acute care settings. Incontinence is a frequent issue in acute care settings. It may affect:
- Patients already incontinent upon admission, especially older adults or those with chronic illnesses.
- Previously continent patients who develop incontinence due to:
-
- Prolonged immobility
- Sedation
- Surgery or acute illness
- Medication side effects
- Neurological impairment
This creates a clinical and operational need to manage urine effectively—balancing hygiene, skin integrity, infection control, and patient dignity.
The Problem
Approaches to urine management in Acute Care.
Indwelling catheterisation
Description
Risks
Main clinical areas
Absorbent Pads/Diapers
Description
Risks
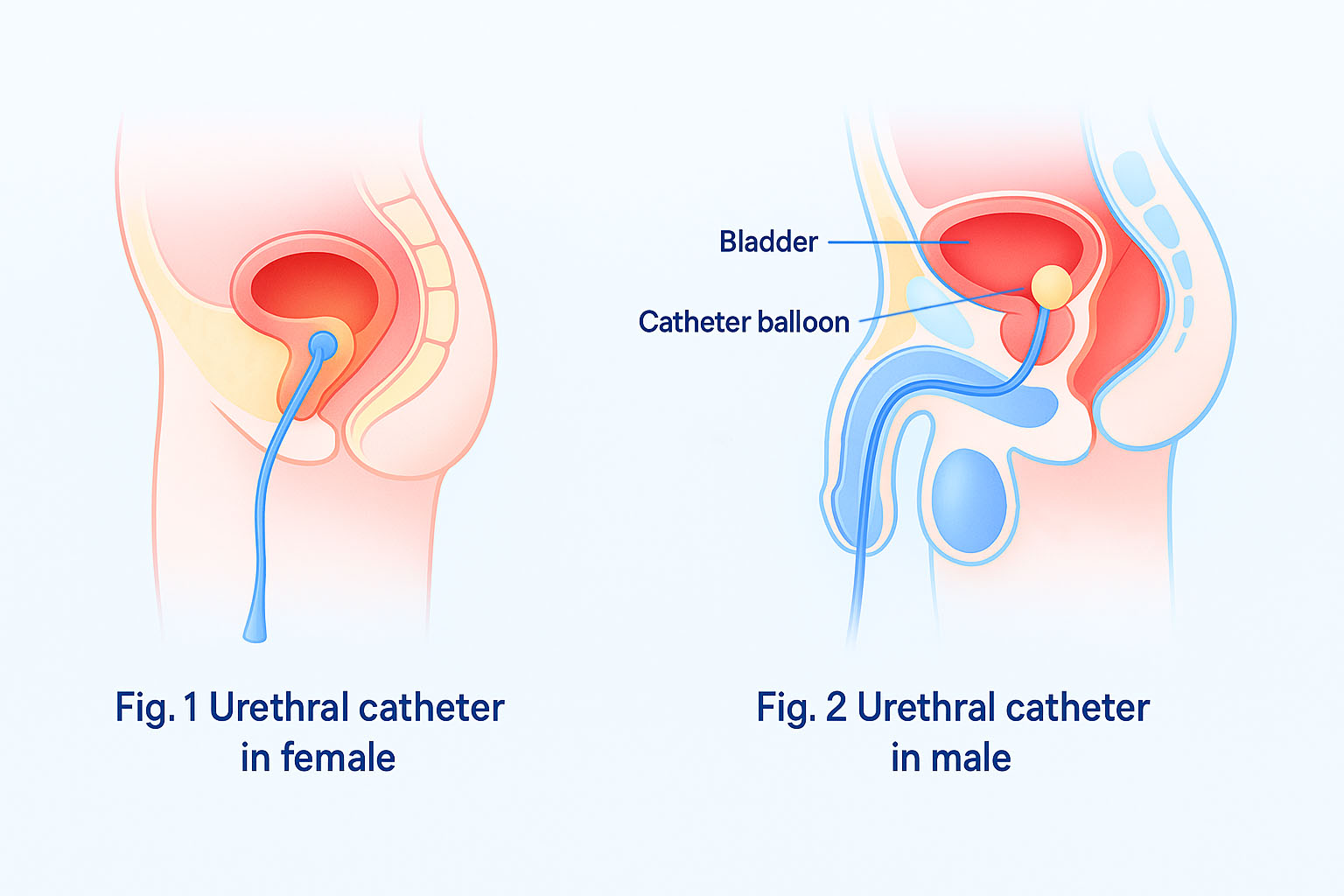
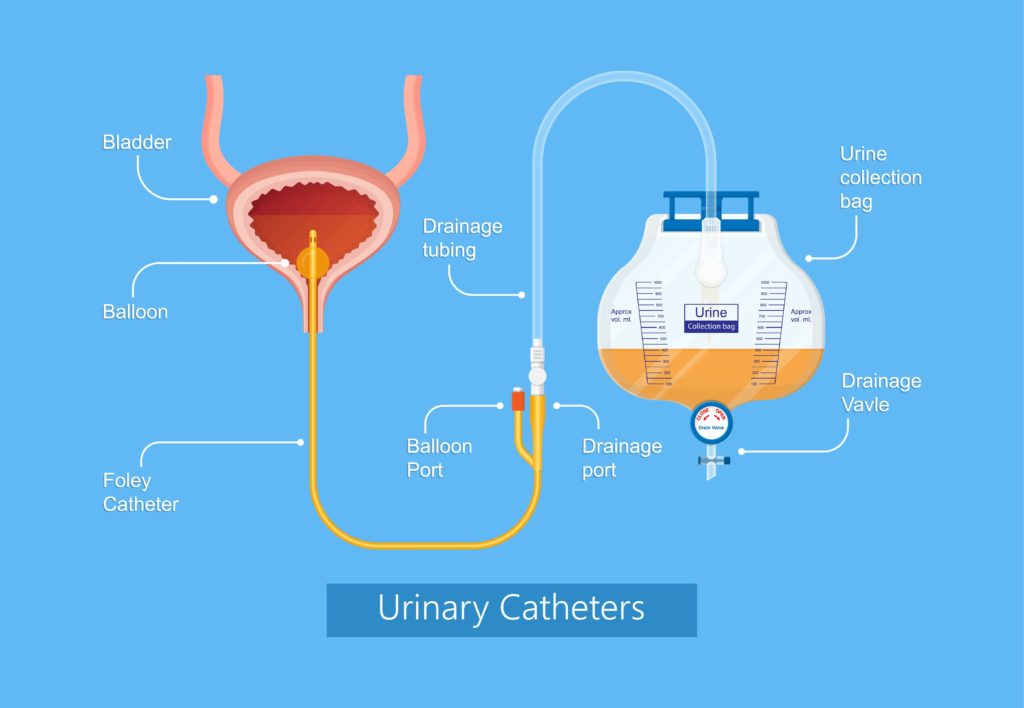
Indwelling urinary catheter (IUC). An indwelling urinary catheter is a flexible tube left in the bladder to drain urine into a collection bag, held in place by a water-filled balloon. It is inserted through the urethra or a small incision in the abdomen (suprapubic catheter) .
Suprapubic catheter. Surgically inserted into the bladder through an incision in the lower abdomen.
The IUC should be inserted only when necessary, and will only be in place as long as indications are met. Indications for an IUC may include, but are not limited to, the following12.
• Perioperative use in selected surgeries (list the selected surgeries) • Acute urinary retention or obstruction
• Hospice, comfort care, or palliative care
• Accurate measurement of urinary output in critically ill patients
• Required strict immobilization for trauma or surgery
A CAUTI occurs when germs (usually bacteria) enter the body through a urinary catheter and cause infection. The infection can happen in any part of the urinary tract (e.g., kidneys, ureters, bladder, and urethra).
How are they caused?
- Bacteria entering the catheter:
The primary cause is bacteria entering the urinary system through the catheter, which is a pathway for germs that are not normally present in the urinary tract.
- Indwelling catheters:
The risk of infection increases with the duration of catheter use; long-term or indwelling catheters carry a higher risk than short-term use.
Symptoms of a CAUTI:
- Lower abdominal pain or groin pain
- High temperature (fever)
- Feeling cold and shivery (chills)
- Confusion
- Foul-smelling or cloudy urine
- Blood in the urine
- Nausea and vomiting
In Europe there are approximately 476.000 CAUTIs1. per year driven by the high use of ICUs, 20,3% of patients have one UCIs during the stay in the hospitals1.
CAUTI implications:
- Morbility and mortality in patients:
- 14,334 deaths in Europe every year 2.
- 2-4 extra incremental length of stay (LOS) days 3.
- 10-30% of Sepsis cases are driven by Urinary Tract Infection (UTIs), mainly CAUTI4.
- AMR:
- One of the highest independent risk for antimicrobial use (adjusted odds ratio ≥2.0) 1.
- Incremental costs for healthcare systems
- 2-4 incremental length of stay, with and average cost of € 1000 per CAUTI3.
- 470 Eur million of annual costs in Europe (estimation)
UTIs and AMR
Antimicrobial resistance (AMR) is a significant problem in Europe. Each year throughout Europe, more than 35,000 people die from infections with bacteria that are resistant to antimicrobials, and 75 % of the total burden of infections with antibiotic-resistant bacteria in Europe was associated with health care.
Resistance to third-generation cephalosporins among Enterobacterales isolates from HAIs was over 40% in seven of 25 countries. Two countries reported over 20% of Enterobacterales isolates resistant to carbapenems with the highest percentages (Romania 42.9% and Greece 40.8%).
AMR = Antimicrobial resistance percentage
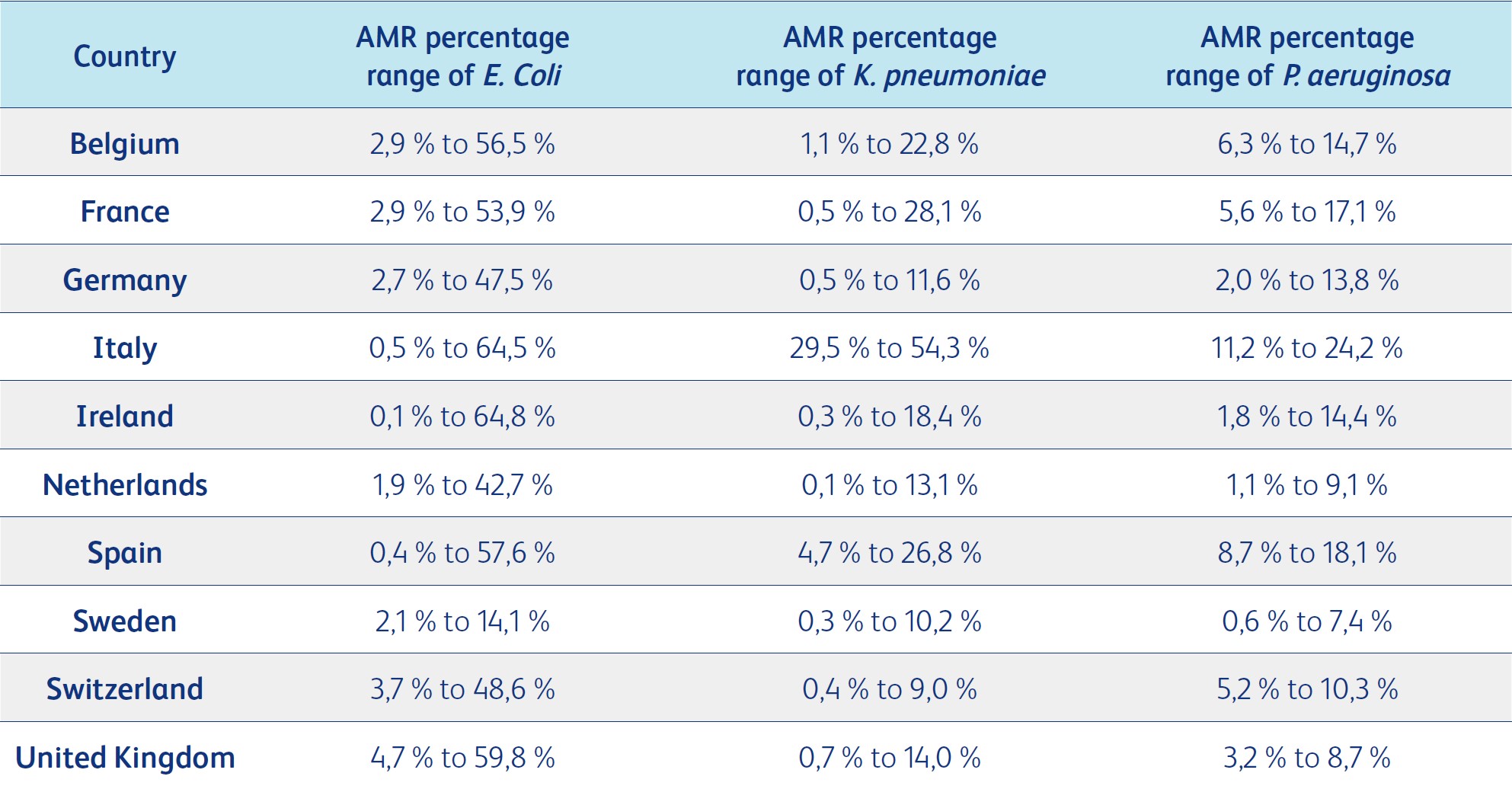
E. coli is the most common cause of community-acquired bloodstream infections and urinary tract infections, with antimicrobial-resistant percentages (percent of isolates that tested resistant to different antimicrobial groups) ranging between 0.2% (Carbapenem) to 54.6% (aminopenicillin) in Europe for various antimicrobial groups. E. coli is followed by K. pneumoniae, with antimicrobial-resistant percentages ranging from 10.0% to 33.9%, and P. aeruginosa, with antimicrobial-resistant percentages ranging from 9.4% to 19.6%. For third-generation cephalosporin resistance in E. coli, countries such as Belgium, France, the Netherlands, Sweden and Switzerland reported the lowest percentages ranging from 5% to 10%. 77
The antimicrobial resistance percentage of third-generation cephalosporin resistance to K. pneumoniae, another common cause of urinary tract infections, varied widely, ranging from less than 1% to more than 50% across Europe. 77
The annual cost of antimicrobial resistance in Europe is around €11.7 billion, or €24 per capita. Around €6.6 billion of the total cost (€13.4 per capita) is related to extra health expenditure from treating resistant infections and their consequences. In addition, €5.1 billion (€10.4 per capita) is related to economic losses due to reduced participation in the workforce (e.g., reduced productivity due to long sick leaves). 76
Due to additional hospital days and treatment costs, a single resistant infection has been estimated to cost about €8,500 to €34,000 more than a non-resistant infection.78
A systematic literature review reported that the attributable costs due to resistant infections were higher compared to susceptible infections, ranging from €303.89 in the UK to €26,357.11 in Germany. The wide variation in costs was related to the type of resistant bacteria studied in different countries, with lower costs associated with E. coli and higher costs associated with Vancomycin-resistant enterococci. The resistant bacteria included E. coli, Klebsiella spp. or Proteus spp., methicillin-resistant Staphylococcus aureus, and Enterobacteriaceae.79
The treatment of CAUTIs contributes to the emerging issue of antibiotic resistance in hospitals, with uropathogens being a leading source of antimicrobial-resistant organisms.34, 61, 62, 80
For Escherichia species, high rates of resistance to ampicillin (87.3%), levofloxacin (81.5%), and nalidixic acid (79.5%) were reported.62
Urinary tract infection is a frequent complication after hip fracture surgery. A 2024 meta-analysis of 42 studies found a pooled postoperative UTI incidence of approximately 11% in geriatric hip fracture patients13. Demographic factors play a role. Women and older adults are consistently found to be at higher risk. In a multihospital hip surgery cohort, female patients had about 2.5 times greater odds of postoperative UTI compared to males14,15. A meta-analysis of hip fracture cases likewise found female gender (OR ≈2.23) and increasing age to be significant risk factors for UTI3.
Neuro Stroke
Stroke patients are particularly vulnerable to CAUTI due to neurologic deficits, impaired bladder function, and prolonged immobilization. Urinary catheterization is commonly used in stroke care for monitoring and managing incontinence, yet it places these patients at increased risk for infection. Studies indicate that stroke patients with a urinary catheter have a 3- to 5-fold increased risk of developing a UTI compared to those managed with alternative strategies16. One observational study demonstrated that stroke patients developing CAUTI had an average hospital stay increase of 3–4 days compared to those without infection. CAUTI can compound neurologic deficits by leading to systemic complications, potentially delaying rehabilitation and recovery. Data from a multicenter study indicate that older age and female sex are associated with a 2- to 3-fold increased risk of CAUTI in the stroke population17.
General wards/Internal medicine
Elderly patients with multiple morbidities represent a particularly vulnerable group when it comes to the use of urinary catheters in general wards. Most general wards patients are elderly with Chronic Heart Failure (CHF), kidney failures and respiratory tract infections.
Chronic Heart Failure (CHF) is the leading cause of hospitalization for those over the age of 65. Its prevalence increases 10-fold from age 60 to age 8018 and 50% of patients with CHF are over 75 years of age19. About half of hospital re-admissions are related to co-morbidities, polypharmacy, and disabilities associated with CHF19. CHF is the leading cause of hospitalization for those over the age of 65 and represents a significant clinical and economic burden.
Occurrence of HF increases with advancing age, and women at older age are at greater risk than men for CHF with preserved ejection fraction19.
Urinary catheterization is commonly used in CHF patients. It facilitates urine output monitoring, which has theoretical benefits in the management of CHF patients with acute volume overload and pulmonary edema. Routine urinary catheterization placement, however, has been associated with unintended consequences of less frequent physical examinations by physicians, less face-to-face time between physicians and patients, and increased rates of UTI20. Apart from the risk of CAUTI, urinary catheter use may contribute to confusion or delirium in some elderly patients, complicating their recovery. Prolonged catheterization can contribute to skin irritation and breakdown, particularly in patients with fragile, aged skin21.
Intensive Care Units (ICU)
The latest ECDC point prevalence survey for HAIs in ICUs (2021)21 reveals that of all patients staying in an ICU for more than two days, 4% presented with urinary tract infection (UTI). And 97% of UTI episodes were associated with presence of a urinary catheter. On average, urinary catheters were used in 89% of the patient-days. The mean device-adjusted rate in patients staying in an ICU for more than two days was 4.4 catheter-associated UTI episodes per 1,000 catheter-days (ICU IQR: 0.7−6.7).
Emergency Departments
The emergency department (ED) is the main entry point for patients admitted to the hospital, where a significant number of urinary catheters (UCs) are placed 23-25.
Although indwelling urinary catheterization is unpleasant and can cause complications, it is often performed without a specific medical indication. Studies indicate that up to 30% of patients in ED receive one urinary catheter without medical indication. Risk factors for patients to receive unnecessary urinary catheters are age (≥70 years), sex (female), co-morbidities and higher illness severity 24,25,26.
One study showed that women were 1.9 times more likely than men, and those aged ≥80 years were 2.9 times more likely than those aged ≤50 years, to have a UC placed without an indication27.
Risks
The use of these absorbent products is associated with several limitations:
- Skin maceration and inflammation can occur due to overhydration from wet absorbent products, resulting in increased skin permeability and susceptibility to irritants.28,29
- The occlusive nature of absorbent pads can change the skin’s microenvironment, potentially leading to increased mechanical irritation, microorganism growth, and immune responses that cause dermatitis symptoms.28
Due to the prolonged contact of urine with the skin, complications can arise with external absorbent products, including IAD and urinary tract infections.28, 29-33
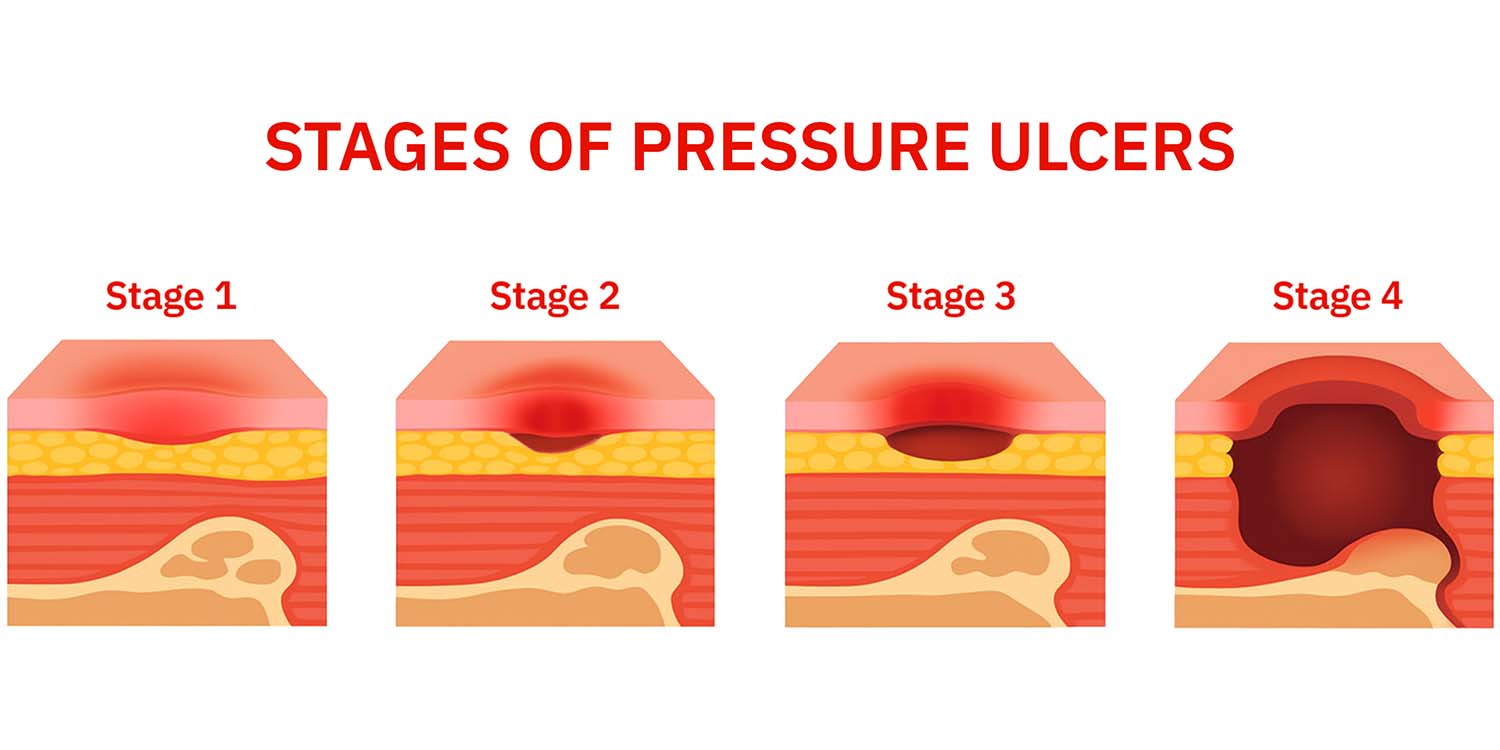
IAD is caused by occlusive containment products, compromised skin, immobility, and poor nutrition. It negatively impacts quality of life, increases susceptibility to secondary infections, and can be challenging, time-consuming, and costly to prevent and treat.
-
- Some of the risk factors for IAD development include the use of occlusive containment products, compromised skin, immobility, inability to perform personal hygiene, medications, poor nutritional status and critical illness.34-36
- Systematic literature reviews and a scoping review reported that IAD is associated with a reduction in the quality of life due to discomfort, burning, itching, and pain in the affected areas, longer lengths of stay, loss of independence, and disrupted activities or sleep.34, 37,38
- The damage to the skin barrier increases patient susceptibility to secondary skin infections.29, 37,38
- Prevention and treatment of IAD can be difficult, time-consuming, and costly.29,34, 39
- The damage to the skin barrier increases patient susceptibility to secondary skin infections.29, 34,35
Data from a big retrospective analysis11 of inpatient data from 15,793,765 patients captured from 937 hospitals shows that patients with IAD stay longer in the hospital, represents higher costs for healthcare settings , experience higher rate of readmissions and higher risk of developing sacral pressure ulcers:
-
- 1,5% of patients admitted in hospitals were incontinent
- IAD prevalence of 0,7%.
- Most incontinent patients (86%) were incontinent of urine only
- Patients treated for IAD were more likely to be immobile and cognitively impaired than those with incontinence only, and received no IAD treatment.
- Compared to continent patients, incontinent patients stayed 2 days longer (4.4 vs 6.4) while LOS was 1.2 days longer for the population older than 75 years (6.3 vs 4.8) and 3.0 days longer for the ICU population (10 vs 7).Statistically significant differences noted for incontinent patients treated for IAD who had 3.3 longer LOS days than incontinent patients without IAD treatment (9.7 days vs 6.4 days). The average LOS was 2.4 days longer in patients 75 years and older who received IAD treatment (8.4 days vs 6.0 days) and 2.9 days longer for the ICU population (12.8 vs 9.9) compared to patients who received no treatment.
- The readmission rate was 1.4 times higher for incontinent patients compared to continent patients (12% vs 8.8%), the 30-day readmission rate was 1.1 times higher in the older population 75 years and older (12% vs 11%), and 1.4 times higher in the ICU population (15% vs 10.9%) .The readmission rate was 1.3 times higher for incontinent patients with IAD treatment compared to incontinent patients without IAD treatment (16% vs 12%), 1.4 times higher in the older population 75 years and older (17% vs 12%), and 1.3 times higher for the ICU population (20% vs 15%).
- The average total index hospital costs were 1.2 times higher for incontinent patients compared to continent patients ($17,020 vs $13,713), 1.3 times higher for incontinent patients with IAD treatment compared to those incontinent patients without IAD treatment ($22,832 vs $16,981) .
- Those with IAD treatment were 2.4 times more likely to have a sacral pressure injury upon admission than continent patients (10.9% vs 4.6%) and 2 times more likely to have a hospital-acquired sacral pressure injury (1.2% vs 0.59%).
Hospital ‐acquired pressure ulcers estimation amounts to 8.4%40. In critical care patients ranges from 26,2 % to 64 %.36,38. In UK data show that pressure ulcers extend hospital stays by 4–10 days (NHS, 2018; Thorpe, 2017) and treat them is a major burden to the UK healthcare sector with costs to the NHS ranging from £0.5–£2.1 billion annually41.
Absorbent pads are porous materials designed to soak up and hold liquids using properties like adhesion and capillary action. Technically, they are made from materials such as cellulose, superabsorbent polymers (SAPs), or non-woven polypropylene that exploit surface tension and the attraction between the liquid and the pad’s fibers to retain moisture. Their construction, including features like dimples or heat-pressed spots, influences their absorption speed and capacity, with variations available for general, oil-specific, or chemical applications.
Material Composition
- Cellulose: Often used in high-quality filter papers or non-woven fabrics for general absorption and use in microbiology.
- Superabsorbent Polymers (SAPs): Integrated into pads to absorb and retain large volumes of liquid relative to their own mass, common in hygiene products.
- Meltblown Polypropylene: A synthetic material used for industrial and hazardous spill absorption, known for being hydrophobic (water-repellent) and effective for oil and chemical spills.
Absorption Mechanism
- Surface Tension: Causes liquid molecules to cohere, pulling them into the pad’s pores.
- Adhesion: The attraction between liquid molecules and the pad’s fibers helps draw and hold the liquid within the material.
- Capillary Action: The combined effect of these forces allows liquid to be drawn into the fine channels of the absorbent material and held there.