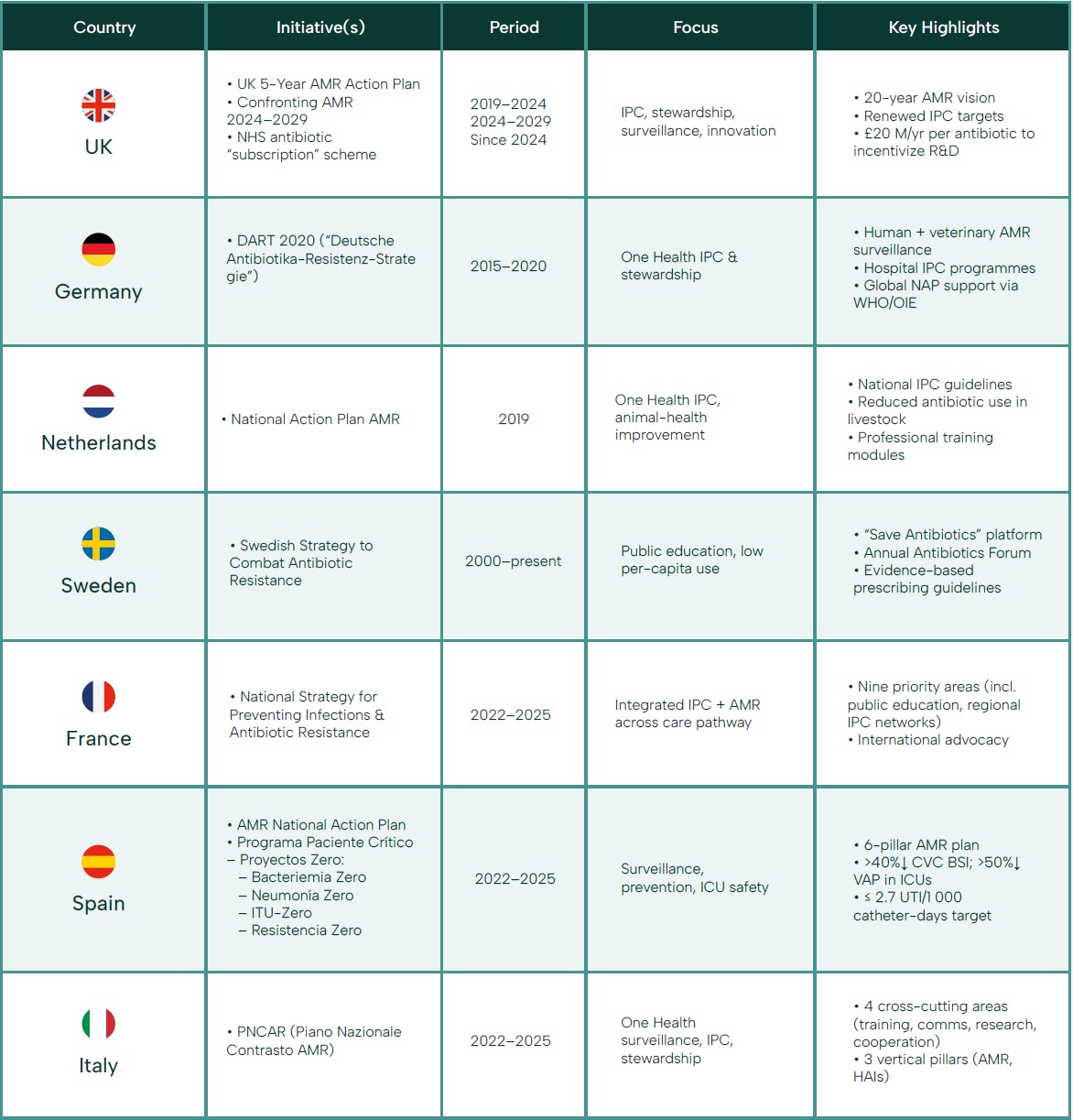
EU Initiatives
CAUTI & IAD prevention effort in Europe
The vast majority of EU countries have no specific CAUTI & IAD focused policies or benchmarks. As a result, data on CAUTI & IAD are limited to point of prevalence surveys or isolated hospital audits, and few countries have set explicit targets to reduce CAUTI & IAD as part of their healthcare quality or AMR plans.
CAUTI iniciatives in Europe
This gap means opportunities for improvement are being missed: without regular measurement or accountability, hospitals may not recognize the scale of their catheter-related infection problems. While some EU Member States are actively working to reduce HAIs, including CAUTIs, there is currently no single, uniform EU-wide target or dedicated plan exclusively for CAUTI reduction. Instead, CAUTI prevention is generally addressed within broader IPC and AMR strategies. ECDC, for example, does not offer standard-alone dedicated guidelines solely in CAUTI prevention.
Main EU-Level Initiatives:
The European Centre for Disease Prevention and Control (ECDC) supports surveillance and provides guidelines on HAIs, including CAUTIs, as part of its broader mandate on IPC. However, the current EU-wide frameworks and funding programs (such as EU4Health) focus on overall HAI reduction and antimicrobial stewardship rather than setting specific quantitative CAUTI reduction targets.
The European One Health Action Plan against AMR, for instance, emphasizes reducing healthcare-associated infections and optimizing catheter use, but it does not include explicit, standalone targets for CAUTI reduction.
National and Regional Strategies:
Several Member States have integrated CAUTI prevention measures into their national IPC guidelines and surveillance systems. For example, countries like the Netherlands and Denmark have
detailed protocols for catheter management and regular monitoring that contribute to CAUTI reduction, though these are typically part of a wider HAI prevention strategy.
Some countries may set internal performance benchmarks or quality indicators related to catheter use and CAUTI rates. However, these targets vary by country and are not standardized across the EU.
In summary, while CAUTI prevention is an important component of existing IPC and AMR policies, specific, standardized EU-wide targets for CAUTI reduction are not yet in place.
CAUTI surveillance systems in Europe
Despite the implications of CAUTI in terms of mortality and morbidity, incremental costs and AMR , dedicated surveillance and prevention efforts for CAUTI are largely lacking in the EU. Unlike surgical site or bloodstream infections, there is no EU-wide system or mandatory program specifically tracking CAUTI rates.
Most EU countries do not have a national CAUTI surveillance network or reduction target. In fact, only a handful of Member States have even issued national guidelines on CAUTI prevention.
In the latest ECDC point of prevalence survey1 the current penetration of surveillance systems and automation in CAUTIs in acute care hospitals was not optimal (right charts).
A few countries (e.g., Spain (EPINE & ENVIN-HELICS, Netherlands (PREZIES), France (RAISIN/SPIADI) have sustained, recurring national-level PPS with CAUTI tracking. Most countries rely heavily on ECDC point of prevelance , conducted every 5 years. ICU-specific surveillance systems (e.g., KISS in Germany) may capture CAUTI data more consistently than general hospital surveillance. In Annex I to this document are described the main point of prevalence studies covering CAUTI by Member States in the EU.
%
do not have a surveillance system
%
have some automation
%
have fully manual surveillance systems
%
have a fully automated surveillance system
IUCs are commonly used but must be applied only when clinically justified, such as:
- Acute urinary retention
- Accurate urine output monitoring in critical care
- Perioperative use in specific surgeries
- Stage III/IV pressure ulcers with incontinence
An ICU should only be placed when there is a clear indication. It should not stay in place longer than necessary. It is important first to consider alternatives before placing an indwelling catheter. A catheter is usually the last resort when other options have failed or proved to be insufficient but may be placed by patient choice in preference to other alternatives. To insert a catheter only for the comfort of the nursing staff and or carers is irresponsible.1
1.-Evidence-based Guidelines for Best Practice in Urological Health Care Indwelling catheterisation in adults Urethral and Suprapubic Alternatives. European Association of Urology Nurses (EAUN) 2024.
The main risks that IUCs drive are the Catheters-related Urinary Tract Infections. (CAUTI). CAUTIs are a major healthcare-associated infection that drive. In Europe there are approximately 476.000 CAUTIs per year driven by the high use of ICUs , 20,3% of patients have one UCIs during the stay in the hospitals.
CAUTI implications:
- Morbility and mortality in patients:
- 14,334 deaths in Europe every year
- 2-4 extra incremental length of stay (LOS) days
- 10-30% of Sepsis cases are driven by Urinary Tract Infection (UTIs) , mainly CAUTI.
- AMR:
- One of the highest independent risk for antimicrobial use (adjusted odds ratio ≥2.0) .
- Incremental costs for healthcare systems
- 470 Eur million of annual costs in Europe (estimation)
CAUTI in the Acute Care
Hip surgery
Urinary tract infection is a frequent complication after hip fracture surgery. A 2024 meta-analysis of 42 studies found a pooled postoperative UTI incidence of approximately 11% in geriatric hip fracture patients2. Demographic factors play
a role. Women and older adults are consistently found to be at higher risk. In a multihospital hip surgery cohort, female patients had about 2.5 times greater odds of postoperative UTI compared to males3. A meta-analysis of hip fracture cases likewise found female gender (OR ≈2.23) and increasing age to be significant risk factors for UTI4.
Neuro Stroke Poner icono en los capítulos sie posible
Stroke patients are particularly vulnerable to CAUTI due to neurologic deficits, impaired bladder function, and prolonged immobilization. Urinary catheterization is commonly used in stroke care for monitoring and managing incontinence, yet it places these patients at increased risk for infection. Studies indicate that stroke patients with a urinary catheter have a to 5-fold increased risk of developing a UTI compared to those managed with alternative strategies5. One observational study demonstrated that stroke patients developing CAUTI had an average hospital stay increase of 3–4 days compared to those without infection. CAUTI can compound neurologic deficits by leading to systemic complications, potentially delaying rehabilitation and recovery. Data from a multicenter study indicate that older age and female sex are associated with a 2- to 3-fold increased risk of CAUTI in the stroke population6.
General wards
: Elderly patients with multiple morbidities represent a particularly vulnerable group when it comes to the use of urinary catheters in general wards. Most of general wards patients are elder patients with Chronic heart failure (CHF) , kidney failures and respiratory tract infections. Chronic heart failure (CHF) is the leading cause of hospitalization for those over the age of 65. Its prevalence increase10 fold from age 60 to age 807 and 50% of patients with CHF are over 75 years of age8 .About half of hospital re-admissions are related to co-morbidities, polypharmacy and disabilities associated with CHF9.CHF is the leading cause of hospitalization for those over the age of 65 and represents a significant clinical and economic burden. Occurrence of HF increases with advancing age, and women at older age are at greater risk than men for CHP with preserved ejection fraction10.
Urinary catheterization is commonly used in CHF patients. It facilitates urine output monitoring, which has theoretical benefits in the management of CHF patients with acute volume overload and pulmonary edema. Routine urinary catheterization placement, however, has been associated with unintended consequences of less frequent physical examinations by physicians, less face-to-face time between physicians and patients, and increased rates of UTI11. Apart of the risk of CAUTI catheter use may contribute to confusion or delirium in some elderly patients, complicating their recovery. Prolonged catheterization can contribute to skin irritation and breakdown, particularly in patients with fragile, aged skin. ICUs: The latest ECDC point prevalence survey for HAIs in ICUs (2021)12 reveals that of all patients staying in an ICU for more than two days, 4% presented with urinary tract infection (UTI). And 97% of UTI episodes were associated with presence of a urinary catheter. On average, urinary catheters were used in 89% of the patient-days. The mean device-adjusted rate in patients staying in an ICU for more than two days was 4.4 catheter-associated UTI episodes per 1 000 catheter-days (ICU IQR:0.7−6.7).
Emergency departments
: The emergency department (ED) is the main entry point for patients admitted to the hospital, where a significant number of urinary catheters (UCs) are placed.2-4
- Gokula RM, Smith MA, Hickner J. Emergency room staff education and use of a urinary catheter indication sheet improves appropriate use of foley catheters. Am J Infect Control 2007;35:589-93. 3. Gokula RR, Hickner JA, Smith MA. Inappropriate use of urinary catheters in elderly patients at a midwestern community teaching hospital. Am J Infect Control 2004;32:196-9. 4. Hazelett SE, Tsai M, Gareri M, Allen K. The association between indwelling urinary catheter use in the elderly and urinary tract infection in acute care. BMC Geriatr 2006;6:1-7
Although indwelling urinary catheterization is unpleasant and can cause complications, it is often performed without a specific medical indication. Studies indicate that up to 30% of patients in ED receive one urinary catheter without medical indication. Risk factors for patients to receive unnecessary urinary catheters are age ( ≥70 years ) . sex (female) , co-morbidities and higher illness severity3.4y 22.
In one study showed that women were 1.9 times more likely than men, and those age ≥80 years were 2.9 times more likely than those age ≤50 years, to have a UC placed without an indication23.
3.Gokula RR, Hickner JA, Smith MA. Inappropriate use of urinary catheters in elderly patients at a midwestern community teaching hospital. Am J Infect Control 2004;32:196-9. 4. Hazelett SE, Tsai M, Gareri M, Allen K. The association between indwelling urinary catheter use in the elderly and urinary tract infection in acute care. BMC Geriatr 2006;6:1-7 22. Holroyd-Leduc JM, Sands LP, Counsell SR, Palmer RM, Kresevic DM, Landefeld CS. Risk factors for indwelling urinary catheterization among older hospitalized patients without a specific medical indication for catheterization. J Patient Saf 2005;1:201-7.
- Mohamad G. Fakih, MD, MPHa,b,c mohamad.fakih@stjohn.org∙ Stephen P. Shemes, BSd ∙ Margarita E. Pena, MDc,e ∙ … ∙ Janice E. Rey, MT (ASCP)b ∙ Susan M. Szpunar, PhDd ∙ Louis D. Saravolatz, MD. Urinary catheters in the emergency department: Very elderly women are at high risk for unnecessary utilization. AJIC Volume 38, Issue 9p683-688November 2010
IAD:
Incontinence-associated dermatitis (IAD), also known as moisture-associated skin damage or irritant contact dermatitis, is a skin condition caused by prolonged exposure to urine or feces. It manifests as redness, swelling, and in some cases, erosion or skin loss. IAD can be painful, itchy, and lead to secondary infections, impacting a person’s quality of life.
IAD is caused by occlusive containment products, compromised skin, immobility, and poor nutrition. It negatively impacts quality of life, increases susceptibility to secondary infections, and can be challenging, time-consuming, and costly to prevent and treat.
Some of the risk factors for IAD development include the use of occlusive
containment products, compromised skin, immobility, inability to perfor
personal hygiene, medications, poor nutritional status and critical illness.182, 183,186
The damage to the skin barrier increases patient susceptibility to secondary skin infections.44, 182, 183
Data from a big retrospective analysis of inpatient data1 from 15,793,765 patients captured from 937 hospitals shows that patients with IAD stay longer in the hospital, represents higher costs for healthcare settings , experience higher rate of readmissions and higher risk of developing sacral pressure ulcers:
- 1,5% of patients admitted in hospitals were incontinent
- IAD prevalence of 0,7%.
- Most incontinent patients (86%) were incontinent of urine only
- Patients treated for IAD were more likely to be immobile and cognitively impaired than those with incontinence only, and received no IAD treatment.
- Compared to continent patients, incontinent patients stayed 2 days longer (4.4 vs 6.4) while LOS was 1.2 days longer for the population older than 75 years (6.3 vs 4.8) and 3.0 days longer for the ICU population (10 vs 7).Statistically significant differences noted for incontinent patients treated for IAD who had 3.3 longer LOS days than incontinent patients without IAD treatment (9.7 days vs 6.4 days). The average LOS was 2.4 days longer in patients 75 years and older who received IAD treatment (8.4 days vs 6.0 days) and 2.9 days longer for the ICU population (12.8 vs 9.9) compared to patients who received no treatment.
- The readmission rate was 1.4 times higher for incontinent patients compared to continent patients (12% vs 8.8%), the 30-day readmission rate was 1.1 times higher in the older population 75 years and older (12% vs 11%), and 1.4 times higher in the ICU population (15% vs 10.9%) .The readmission rate was 1.3 times higher for incontinent patients with IAD treatment compared to incontinent patients without IAD treatment (16% vs 12%), 1.4 times higher in the older population 75 years and older (17% vs 12%), and 1.3 times higher for the ICU population (20% vs 15%).
- The average total index hospital costs were 1.2 times higher for incontinent patients compared to continent patients ($17,020 vs $13,713), 1.3 times higher for incontinent patients with IAD treatment compared to those incontinent patients without IAD treatment ($22,832 vs $16,981) .
- Those with IAD treatment were 2.4 times more likely to have a sacral pressure injury upon admission than continent patients (10.9% vs 4.6%) and 2 times more likely to have a hospital-acquired sacral pressure injury (1.2% vs 0.59%).
Hospital ‐acquired pressure ulcers estimation amounts to 8.4%6. In critical care patients ranges from 26,2 % to 64 %.185,186. In UK data show that pressure ulcers extend hospital stays by 4–10 days (NHS, 2018; Thorpe, 2017) and treat them is a major burden to the UK healthcare sector with costs to the NHS ranging from £0.5–£2.1 billion annually (Bennett et al., 2004; Dealey et al., 2012; Guest et al., 2017, 2018a, 2018b).
Higher rates of UTI in women
Many of patients admitted in emergency departments hospitals had one UTI as a result of the use of diapers. In one retrospective cohort study in one emergency department 30% of patients admitted to hospitals had a urinary tract infection in the emergency department. The use of absorbent incontinence pads was associated with a higher probability of urinary tract infection at admission. The probability was even higher amongst patients with permanent catheters at admission :full-time users of absorbent incontinence pads had a higher probability of being admitted with urinary tract infection (Odds Ratio=2.00 (95% Confidence Interval: 1.61–2.49); p<.001). Patients identified as severely frail had a higher probability of becoming pad users during hospitalization (Odds Ratio=1.57 (95% Confidence Interval: 1.45–1.71); p<.001) compared to non/mild/moderate frail patients. 1 The study shows that patients who became pad users during hospitalization had a higher risk of a hospital-acquired urinary tract infection (Odds Ratio=4.28 (95% Confidence Interval: 1.92–9.52); p<.001).
In the case of women, anatomical factors and alterations also play an important role in the pathogenesis of UTI in women. The shortness of the urethra, with its close relationship to the anus, makes it easy for bacteria to ascend in the urinary tract. In women, fecal-perineal-urethral contamination is the most probable explanation for infections caused by enteric bacteria, as demonstrated by experiments evaluating the genotype of E. coli strains causing UTI in women.2,3
In a case-control study, 100 women with recurrent UTI and 113 controls were investigated to determine whether there were differences in perineal anatomic measurements, postvoid residual urine volume, or in urine voiding characteristics. The distance from the urethra to the anus was significantly shorter in cases than in controls (4.8 cm and 5.0 cm, respectively, P = 0.03). No other differences were identified between cases and controls. These data suggest that pelvic anatomical characteristics may play a role in predisposing young women to recurrent UTI, especially those who do not have exogenous risk factors for these infections.4
- Absorbent incontinence pad use and the association with urinary tract infection and frailty: A retrospective cohort study Emma Bendix Larsen , Caroline Lunne Fahnøe , Peter Errboe Jensen , Merete Gregersen
2.Yamamoto S, Tsukamoto T, Terai A, Kurazono H, Takeda Y, Yoshida O. Genetic evidence supporting the fecal-perineal-urethral hypothesis in cystitis caused by Escherichia coli. J Urol. 1997;157(3):1127–1129. [PubMed] [Google Scholar]
3.Mitsumori K, Terai A, Yamamoto S, Yoshida O. Virulence characteristics and DNA fingerprints of Escherichia coli isolated from women with acute uncomplicated pyelonephritis. J Urol. 1997;158(6):2329–2332. doi: 10.1016/s0022-5347(01)68244-2. [DOI] [PubMed] [Google Scholar]
4.Hooton TM, Stapleton AE, Roberts PL, et al. Perineal anatomy and urine-voiding characteristics of young women with and without recurrent urinary tract infections. Clin Infect Dis. 1999;29(6):1600–1601. doi: 10.1086/313528. [DOI] [PubMed] [Google Scholar]